
|

|

|

|
Tint etching is particularly well suited for copper and copper alloys. Klemm's I reagent works very well with most of these compositions. Beraha's lead sulfide tint etch is also useful. All of the copper samples to be shown were attack polished using 1% aqueous ferric nitrate added to colloidal silica. Coloration is generally somewhat different, and sometimes less sharp, when other polishing abrasives are used.

|

|

|

|
Figure 1 (above) shows the microstructure of cold worked and annealed alpha brass (Cu-30% Zn) and three examples of alpha-beta brass (Cu-40% Zn) heat treated in various ways to alter the grain size and amount and distribution of the phases. For the alpha brass sample (A), etching is very slow -- requiring nearly an hour. Tint etching has revealed all of the grains and the annealing twins. With ordinary chemical etchants it is very difficult to get such complete revelation of the grain structure.9

|
For the alpha-beta brass samples, the anodic beta phase is colored rather quickly, generally less than 5 min is required. The sample (B) heated to 940 F (505 C) and water quenched contains close to the minimum amount of beta (actually, ordered beta) that will form. Tint etching produces only a light yellowish color in all of the beta grains. |

|
Samples heated higher in the two phase region -- 1200 F (650 C) and water quenched -- contain more beta which is tinted with a variety of colors depending on orientation. |

|
The third example -- heated into the all-beta region and air cooled -- shows three prior beta grains where the beta phase within each of these regions has the same color. Note that the coloring is very precise, no enlargement of the beta occurs, and all of the beta is revealed. None of these samples was pre-etched -- the attack polish produced enough relief to eliminate need for one. |

|
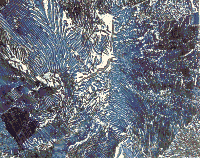
Figure 2 shows the microstructure of eutectic Al-33% Cu tint etched with the aqueous ammonium molybdate-ammonium chloride solution suggested by Lienard and Pacque.10 This sample was attack polished using a few mL of 0.5% aqueous HF added to colloidal silica in the Fini-Pol device. The CuAl2 phase is colored violet. |
|
Figure 3 illustrates the use of Beraha's acidified aqueous sodium molybdate reagent to color the cathodic cementite in an Fe- 1% C, high purity alloy where the cementite in the pearlite is blue but the grain boundary cementite is violet. |

|
Figure 4 shows the microstructure of solution annealed, austenitic Hadfield manganese steel. After polishing with colloidal silica, the sample was given a light pre-etch with 2% nital (3 s) and then etched 20 s with 20% aqueous sodium metabisulfite. This procedure is also effective for revealing the a + e structure of decarburized surfaces of the alloy.11 |
Tint etched samples can also be photographed in black and white and still produce superb results (see Fig. 5 and 6).

|

|
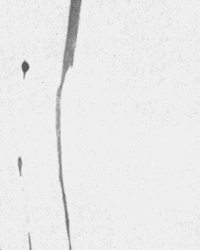
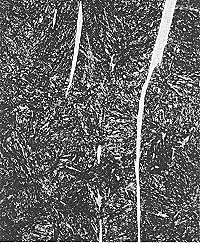
| Figure 5 (above)-- Tint etching of nickel base superalloys such as X750 using Beraha's reagent (right) can produce excellent color contrast development of twin and grain structures compared with that produced using standard reagents such as Kalling's No.2 etch (left). B&W photos clearly reveal color differences. 100X. Composition of Beraha's tint etchant: 100mL HCL, 50 mL H2O,1 g potassium metabisulfite, and 1 g ferric chloride. |
|
|
| Figure 6 (above)-- Beraha's lead sulfide etch will color sulfide inclusions white. Shown here is quenched and tempered AISI 41S50, pre-etched with 2% nital. Photomicrograph at left is of as-polished steel; one at right is of the tint etched sample. 500X |
1. Color Metallography, by E. Beraha and B. Shpigler: American
Society for Metals, Metals Park, Ohio, 1977.
2. Metallography: Principles and Practice, by G. F. Vander Voort:
McGraw-Hill Book Co., New York, 1984.
3. "The Mechanism of Metallographic Etching," by G.L. Kehl and M. Metlay:
Journal of the Electrochemical Society, Vol 101, March 1954, p
124-127.
4. "Metallographic Reagents Based on Sulfide Films," by E. Beraha:
Praktische Metallographie, Vol 7, 1970, p 242-248.
5. "Metallographic Reagents Based on Molybdate Solutions," by E. Beraha:
Praktische Metallographie, Vol 11, 1974, p 271-275.
6. Supplies Ltd., 6272 W. North Ave., Chicago Il. 60639.
7. Buehler Ltd.,41 Waukegan Rd.,Lake Bluff, Il. 60044.
8. Struers Inc., 20102 Progress Dr., Cleveland, Ohio 44136.
9. "An Improved Etchant for Copper and Copper Alloys," by R. M. Slepian
and J. P. Prohaska: Metallography, Vol 9, 1976, p 51-61.
10. "Analysis of the Selective Coloring Mechanism for Identification of
Different Phases in Al-Si-Cu Foundry Alloys," by P. Lienard and C. Pacque:
Homnes Fonderie, Vol 126, June-July 1982, p 27-35.
11. "Austenitic Manganese Steel: Structure and Properties of Decarburized
Layer," by A. J. Sedriks and T. O. Mulhearn: J.I.S.I., Vol 202, November
1964, p 907-911.
[Back to Tint Etching] |
[AM&P Articles]
|
[Home] |
[Metallography FAQ] |
|---|